CRISPR-Mediated Whole Gene Transfer: A Powerful New Tool
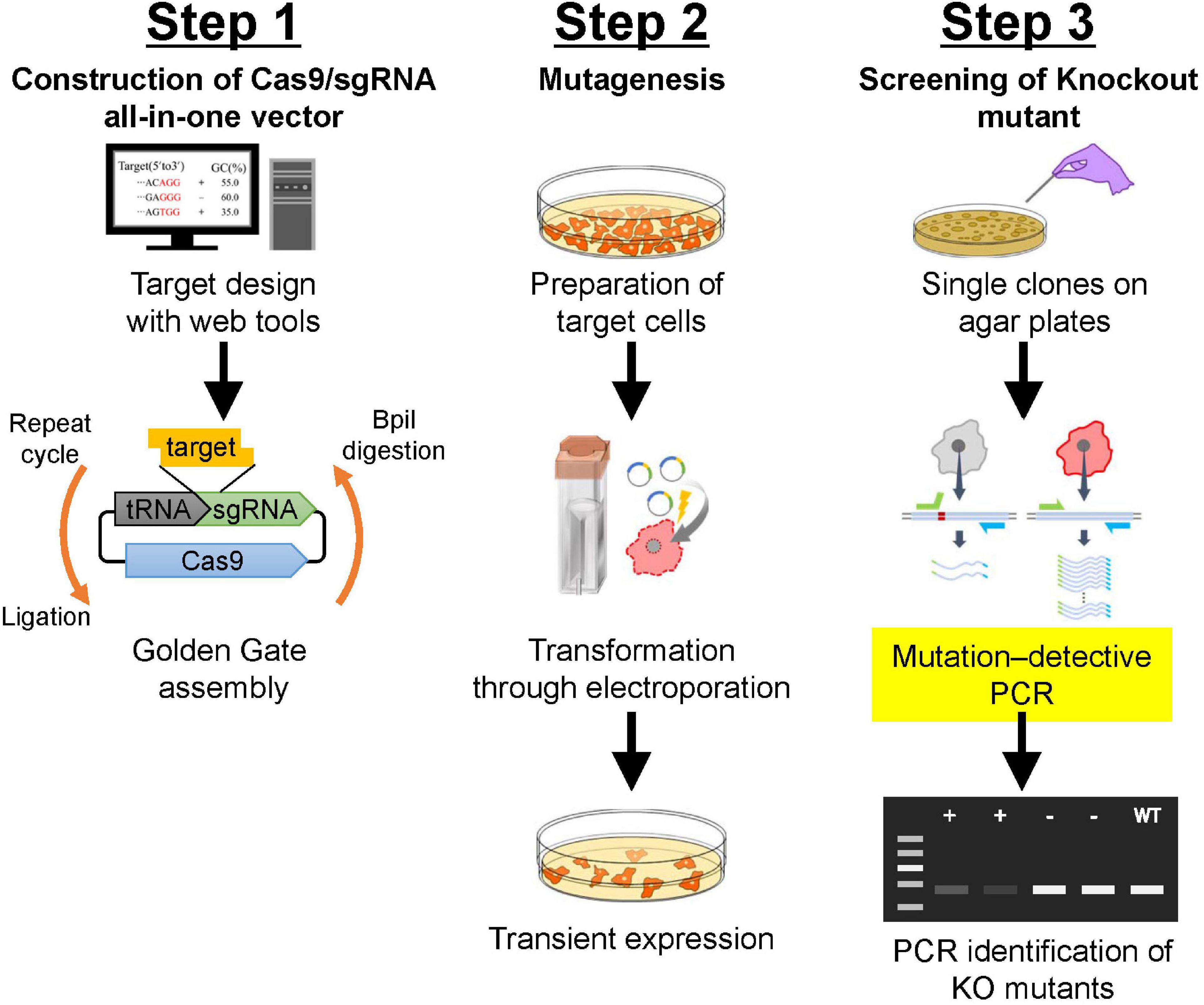
Table of Contents
Mechanism of CRISPR-Mediated Whole Gene Transfer
Understanding CRISPR-Cas Systems
The CRISPR-Cas system, derived from bacteria's natural defense mechanism against viruses, is the core of this technology. It utilizes a guide RNA (gRNA) molecule, designed to be complementary to a specific DNA sequence, to target a desired gene. This gRNA molecule is coupled with a Cas enzyme, most commonly Cas9, but also including variants like Cas12a and Cas13d, which acts as molecular scissors, cleaving the DNA at the targeted location. This DNA cleavage initiates a cellular repair process, either through non-homologous end joining (NHEJ), which often introduces insertions or deletions, or homology-directed repair (HDR), which allows for the precise insertion of a new gene.
- Step-by-step process:
- Design a gRNA specific to the target gene.
- Deliver the gRNA and Cas enzyme into the target cell.
- Cas enzyme creates a double-stranded break at the target site.
- The cell repairs the break using either NHEJ or HDR.
- In HDR, a donor DNA template containing the desired gene is provided, enabling precise gene replacement. This is crucial for whole gene transfer.
- Different Cas enzymes offer varying advantages; Cas9 is widely used, while Cas12a offers enhanced specificity in some applications.
Delivery Methods for Whole Gene Transfer
Efficient delivery of the CRISPR-Cas system and the new gene is crucial for successful whole gene transfer. Several methods are employed, each with its advantages and disadvantages:
-
Viral vectors: Lentiviruses and adeno-associated viruses (AAVs) are commonly used due to their high efficiency in delivering genetic material into cells. However, viral vectors can trigger immune responses and have size limitations.
-
Non-viral methods: Lipid nanoparticles (LNPs) and electroporation are less immunogenic than viral vectors but often have lower transfection efficiency. LNPs are particularly promising for delivering CRISPR components to specific tissues. Electroporation involves using electric pulses to create temporary pores in cell membranes, allowing entry of the CRISPR components.
-
Comparison of delivery methods:
| Method | Efficiency | Safety | Target Cell Types | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Viral vectors | High | Moderate | Wide range | High transduction efficiency | Immunogenicity, size limitations, potential toxicity |
| Lipid nanoparticles | Moderate | High | Specific tissues | Less immunogenic, versatile | Lower transfection efficiency than viral vectors |
| Electroporation | Moderate | Moderate | Varies | Relatively simple, versatile | Can cause cell damage, lower efficiency |
Applications of CRISPR-Mediated Whole Gene Transfer
Gene Therapy
CRISPR-mediated whole gene transfer is revolutionizing gene therapy by allowing the precise replacement of faulty genes responsible for inherited diseases. This offers a potential cure rather than just symptom management.
- Examples of applications:
- Cystic fibrosis: Replacing the faulty CFTR gene.
- Sickle cell anemia: Correcting the mutation in the β-globin gene.
- Hemophilia: Introducing functional clotting factor genes.
Clinical trials are ongoing for several genetic diseases, showing promising results and paving the way for wider applications.
Agricultural Biotechnology
CRISPR technology enhances crop improvement by enabling precise modifications to introduce desirable traits.
- Examples:
- Increased crop yields: Enhancing photosynthesis efficiency or stress tolerance.
- Pest resistance: Introducing genes that provide natural pest defense mechanisms.
- Improved nutritional value: Enhancing vitamin or mineral content.
Ethical considerations and public perception surrounding genetically modified organisms (GMOs) remain important factors influencing the adoption of this technology in agriculture.
Biomanufacturing
CRISPR-mediated whole gene transfer enables the engineering of cells to produce therapeutic proteins or other valuable compounds at a higher yield and more efficiently.
- Examples:
- Production of therapeutic antibodies: Engineering cells to secrete large quantities of monoclonal antibodies for treating various diseases.
- Production of enzymes: Engineering microbes to produce enzymes for industrial applications.
- Production of biofuels: Engineering algae or other microorganisms to produce biofuels.
This approach offers potential for increased scalability and cost-effectiveness compared to traditional biomanufacturing methods.
Challenges and Future Directions of CRISPR-Mediated Whole Gene Transfer
Off-target Effects
One of the major challenges is the potential for off-target effects – unintended edits at non-target sites in the genome. These off-target mutations could have harmful consequences.
- Mitigation strategies:
- Careful design of guide RNAs to enhance specificity.
- Utilizing improved Cas enzymes with higher fidelity.
- Employing multiple guide RNAs to target different regions of the gene.
- Developing sophisticated screening methods to detect off-target effects.
Ethical Considerations
The power of CRISPR-mediated whole gene transfer necessitates careful consideration of ethical implications, particularly regarding germline editing – modifying genes in reproductive cells that are passed on to future generations. The potential for unintended consequences and societal impact must be carefully weighed.
- Responsible research practices: Establishing strict guidelines and oversight for research involving germline editing is critical.
- Public engagement and education: Fostering open discussions about the ethical considerations surrounding this technology is essential.
Further Research and Development
Ongoing research focuses on improving the efficiency, safety, and delivery methods of CRISPR-mediated whole gene transfer.
- Areas of active research:
- Developing novel Cas enzymes with enhanced specificity and reduced off-target effects.
- Improving the efficiency and safety of delivery systems.
- Exploring new applications of the technology, such as in cancer therapy and regenerative medicine.
- Developing more sophisticated computational tools for guide RNA design and off-target prediction.
Conclusion
CRISPR-mediated whole gene transfer is transforming the field of genetic engineering, offering unprecedented opportunities to treat diseases, improve crops, and develop new biotechnologies. While challenges remain, particularly regarding off-target effects and ethical considerations, ongoing research is paving the way for safer and more efficient applications. The future of medicine, agriculture, and biotechnology is inextricably linked to the continued development and responsible implementation of CRISPR-mediated whole gene transfer technology. Learn more about the exciting advancements in this field and explore the potential of CRISPR gene editing to shape a healthier and more sustainable future.

Featured Posts
-
 Is Jacob Alons Fairy In A Bottle The Next Big Hit
May 30, 2025
Is Jacob Alons Fairy In A Bottle The Next Big Hit
May 30, 2025 -
 Revolucion En La Compra De Boletos Ticketmaster Presenta Virtual Venue
May 30, 2025
Revolucion En La Compra De Boletos Ticketmaster Presenta Virtual Venue
May 30, 2025 -
 Solicita Tu Reembolso Cancelacion Del Festival Axe Ceremonia 2025 En Ticketmaster
May 30, 2025
Solicita Tu Reembolso Cancelacion Del Festival Axe Ceremonia 2025 En Ticketmaster
May 30, 2025 -
 Selena Gomez Achieves Top 10 Without An Official Single
May 30, 2025
Selena Gomez Achieves Top 10 Without An Official Single
May 30, 2025 -
 Journalists Under Threat Reporting On Bolle Joss Drug Trafficking In Sierra Leone
May 30, 2025
Journalists Under Threat Reporting On Bolle Joss Drug Trafficking In Sierra Leone
May 30, 2025
