Sanofi's Chlamydia Vaccine: A Step Closer To Approval With FDA Fast Track Designation
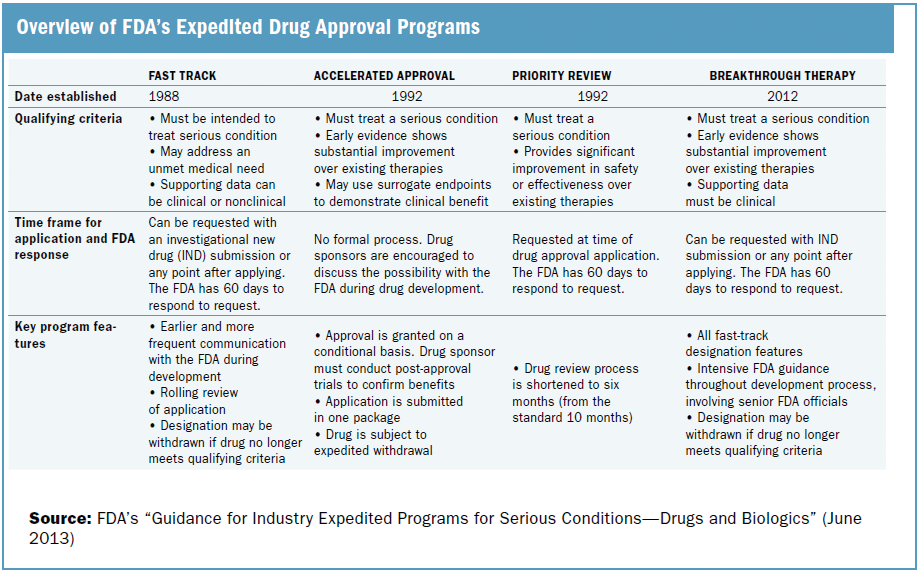
Table of Contents
The Urgent Need for a Chlamydia Vaccine
Chlamydia infections represent a considerable public health crisis. The World Health Organization (WHO) estimates millions of new cases annually, highlighting the urgent need for effective prevention strategies. Chlamydia trachomatis, the bacterium responsible for this STI, often presents asymptomatically, leading to delayed diagnosis and treatment. This silent spread contributes to its widespread prevalence and long-term health consequences.
The challenges of current chlamydia treatment methods further underscore the critical need for a vaccine. These challenges include:
- High infection rates: Chlamydia is the most commonly reported STI in many countries, with disproportionately high rates among young adults.
- Long-term health consequences: Untreated chlamydia can lead to serious complications such as pelvic inflammatory disease (PID), ectopic pregnancy, and infertility in women, and epididymitis in men.
- Antibiotic resistance: The increasing prevalence of antibiotic-resistant strains of C. trachomatis necessitates alternative preventative measures. Treatment failure due to resistance further highlights the pressing need for a vaccine.
Sanofi's Chlamydia Vaccine Candidate: Key Features and Development
Sanofi's chlamydia vaccine candidate represents a significant advancement in STI vaccine development. While specific details about the vaccine's mechanism of action may be proprietary, its progress through clinical trials demonstrates promising results. This vaccine candidate stands apart due to:
- Innovative Technology Platform: The exact technology platform remains undisclosed, but its successful progression through clinical trials suggests an effective immune response generation. This platform likely focuses on eliciting robust and long-lasting immunity.
- Promising Clinical Trial Results: The published data from clinical trials, though not yet fully public, has demonstrated acceptable efficacy and safety profiles. Further details on the immune response elicited and duration of protection are expected as more data emerges.
- Targeted Approach: The vaccine likely targets specific antigens on C. trachomatis to minimize side effects while maximizing effectiveness.
FDA Fast Track Designation: Implications and Accelerated Approval Process
The FDA's fast-track designation is a significant milestone for Sanofi's chlamydia vaccine. This designation is granted to expedite the development and review of drugs that treat serious conditions and address unmet medical needs. For Sanofi's vaccine, this means:
- Prioritized Review: Sanofi will receive increased access to FDA guidance and support throughout the development process.
- Accelerated Clinical Trial Design: The FDA may allow for more efficient clinical trial design and conduct, reducing the time to market.
- Potential for Earlier Approval: If the vaccine meets all regulatory requirements, the fast-track designation could significantly shorten the overall timeline for approval and market launch.
Potential Impact on Public Health and Disease Prevention
The successful development and widespread adoption of Sanofi's chlamydia vaccine have the potential to revolutionize chlamydia prevention and public health strategies. The potential impact includes:
- Reduced Infection Rates: A highly effective vaccine could dramatically reduce the incidence of chlamydia infections globally, particularly among high-risk populations.
- Improved Reproductive Health: Lower infection rates translate to fewer cases of PID, ectopic pregnancy, and infertility, leading to improved reproductive health outcomes.
- Decreased Healthcare Costs: The reduction in chlamydia infections would lessen the burden on healthcare systems, reducing the costs associated with diagnosis, treatment, and management of complications.
- Cost-Effectiveness of Vaccination Programs: Considering the high prevalence and associated healthcare costs of chlamydia, a vaccination program could be highly cost-effective in the long term.
Conclusion
Sanofi's chlamydia vaccine, with its FDA fast-track designation, represents a critical advancement in the fight against this pervasive STI. The potential public health impact is substantial, promising a significant reduction in infection rates and improved sexual health outcomes. This breakthrough showcases the power of innovative vaccine development and the crucial role of regulatory pathways in accelerating the availability of life-improving medical interventions. Stay updated on the latest developments regarding Sanofi's chlamydia vaccine and join the fight against this prevalent STI. Learn more about chlamydia prevention and related resources on the [link to Sanofi's website] and the [link to the FDA's website].

Featured Posts
-
 The Comprehensive Summer Arts And Entertainment Guide For City Region
May 31, 2025
The Comprehensive Summer Arts And Entertainment Guide For City Region
May 31, 2025 -
 Team Victoriouss Preparation For The Tour Of The Alps
May 31, 2025
Team Victoriouss Preparation For The Tour Of The Alps
May 31, 2025 -
 Are New Covid Variants Ba 1 And Lf 7 A Threat In India Insacog Data Provides Insights
May 31, 2025
Are New Covid Variants Ba 1 And Lf 7 A Threat In India Insacog Data Provides Insights
May 31, 2025 -
 Grigor Dimitrov Ochakvaniyata Za Rolan Garos 2024
May 31, 2025
Grigor Dimitrov Ochakvaniyata Za Rolan Garos 2024
May 31, 2025 -
 Finding The Good Life A Balanced Approach To Happiness And Success
May 31, 2025
Finding The Good Life A Balanced Approach To Happiness And Success
May 31, 2025
